Difference between revisions of "Chemical Oxidation (In Situ - ISCO)"
m (1 revision imported) |
m (1 revision imported) |
||
| Line 19: | Line 19: | ||
[[wikipedia: In situ | In situ]] chemical oxidation (ISCO) is a mature technology for remediation of contaminated groundwater, including both source zones and contaminant plumes. ISCO involves the introduction of chemical oxidants into the subsurface to react with contaminants to convert them into less harmful products. Commonly used oxidants include [[wikipedia: Fenton's reagent | Fenton’s reagent]], [[wikipedia: Ozone | ozone]], [[wikipedia: Potassium permanganate | potassium permanganate]], and [[wikipedia: Sodium persulfate | sodium persulfate]]. | [[wikipedia: In situ | In situ]] chemical oxidation (ISCO) is a mature technology for remediation of contaminated groundwater, including both source zones and contaminant plumes. ISCO involves the introduction of chemical oxidants into the subsurface to react with contaminants to convert them into less harmful products. Commonly used oxidants include [[wikipedia: Fenton's reagent | Fenton’s reagent]], [[wikipedia: Ozone | ozone]], [[wikipedia: Potassium permanganate | potassium permanganate]], and [[wikipedia: Sodium persulfate | sodium persulfate]]. | ||
| − | Treatment objectives for ISCO have ranged from reducing contaminant mass within a source zone to meeting maximum contaminant levels (MCLs) in a plume. The effectiveness of ISCO varies as it is highly dependent on proper site characterization, [[Chemical Oxidation Design Considerations(In Situ - ISCO)|ISCO design considerations]], and oxidant delivery system design<ref>Siegrist, R. L. Urynowicz, M.A., West, O.R., Crimi, M.L. and Lowe, K.S., 2001. Principles and practices of in situ chemical oxidation using permanganate. Columbus, OH: Battelle Press. ISBN-10: 1574771027. [http://dx.doi.org/10.1016/s0304-3894(01)00355-7 doi: 10.1016/S0304-3894(01)00355-7]</ref><ref>ITRC, 2005. Technical and Regulatory Guidance for In Situ Chemical Oxidation of Contaminated Soil and Groundwater. Council TITaR, editor. [ | + | Treatment objectives for ISCO have ranged from reducing contaminant mass within a source zone to meeting maximum contaminant levels (MCLs) in a plume. The effectiveness of ISCO varies as it is highly dependent on proper site characterization, [[Chemical Oxidation Design Considerations(In Situ - ISCO)|ISCO design considerations]], and oxidant delivery system design<ref>Siegrist, R. L. Urynowicz, M.A., West, O.R., Crimi, M.L. and Lowe, K.S., 2001. Principles and practices of in situ chemical oxidation using permanganate. Columbus, OH: Battelle Press. ISBN-10: 1574771027. [http://dx.doi.org/10.1016/s0304-3894(01)00355-7 doi: 10.1016/S0304-3894(01)00355-7]</ref><ref>ITRC, 2005. Technical and Regulatory Guidance for In Situ Chemical Oxidation of Contaminated Soil and Groundwater. Council TITaR, editor. [[Media:ITRC-2005-Tech_and_Reg_Guidance.pdf|Report pdf]]</ref><ref>Huling, S. G., and Pivetz, B. E., 2006. In-situ chemical oxidation (No. EPA/600/R-06/072). Environmental Protection Agency, Washington, DC. Office of Water. [[Media:Huling-EPA-ISCO.pdf|Report pdf]]</ref><ref>Krembs, F.J., Siegrist, R.L., Crimi, M.L., Furrer, R.F. and Petri, B.G., 2010. ISCO for groundwater remediation: analysis of field applications and performance. Groundwater Monitoring & Remediation, 30(4), 42-53. [http://dx.doi.org/10.1111/j.1745-6592.2010.01312.x doi: 10.1111/j.1745-6592.2010.01312.x]</ref><ref name ="Siegrist2011">Siegrist, R.L., Crimi, M. and Simpkin, T.J. eds., 2011. In situ chemical oxidation for groundwater remediation (Vol. 3). Springer Science & Business Media. 678 pgs. ISBN: 978-1-4419-7825-7. [http://dx.doi.org/10.1007/978-1-4419-7826-4 doi: 10.1007/978-1-4419-7826-4]</ref><ref name="McGuire2016">McGuire, T., 2016. Development of an Expanded, High-Reliability Cost and Performance Database for In-Situ Remediation Technologies. ESTCP Project ER-201120. [https://serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-201120/ER-201120 ER-201120]</ref>. |
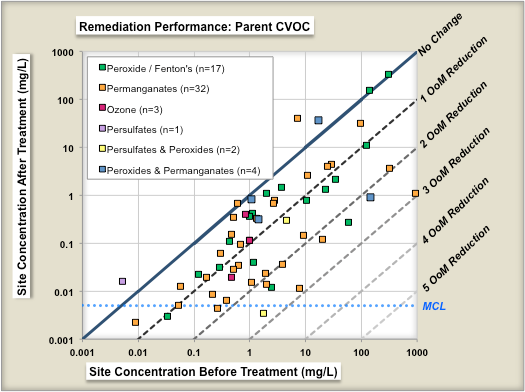
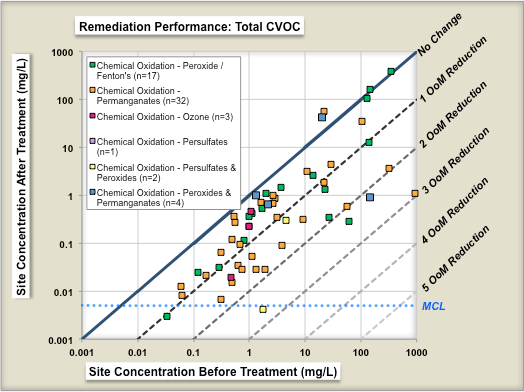
One study evaluated the performance of 70 chemical oxidation projects at [[Chlorinated Solvents | chlorinated solvent]] sites in terms of the reduction in source concentrations before and after treatment<ref name="McGuire2016"/>. Figure 1 shows change in geometric means of parent compound (left panel) and change in geometric means of Total CVOC compound concentrations (right panel) as a result of ISCO treatment. Each symbol is an individual ''in situ'' remediation project at a specific site. The geometric mean of the before-treatment zone concentration is shown on the X-axis, and the after-treatment zone concentration is shown on the Y-axis. The different colored symbols represent different ISCO technology subtypes. The median project was able to reduce the parent compound concentrations in the treatment zone by ~84% (0.8 Orders of Magnitude or OoMs). | One study evaluated the performance of 70 chemical oxidation projects at [[Chlorinated Solvents | chlorinated solvent]] sites in terms of the reduction in source concentrations before and after treatment<ref name="McGuire2016"/>. Figure 1 shows change in geometric means of parent compound (left panel) and change in geometric means of Total CVOC compound concentrations (right panel) as a result of ISCO treatment. Each symbol is an individual ''in situ'' remediation project at a specific site. The geometric mean of the before-treatment zone concentration is shown on the X-axis, and the after-treatment zone concentration is shown on the Y-axis. The different colored symbols represent different ISCO technology subtypes. The median project was able to reduce the parent compound concentrations in the treatment zone by ~84% (0.8 Orders of Magnitude or OoMs). | ||
| Line 47: | Line 47: | ||
Siegrist et al. (2011)<ref name ="Siegrist2011" /> provides a comprehensive description of principles and practices of ISCO for remediation of contaminated groundwater. Tools available to aid with screening, selecting, and implementing ISCO for remediation of contaminated groundwater, include: | Siegrist et al. (2011)<ref name ="Siegrist2011" /> provides a comprehensive description of principles and practices of ISCO for remediation of contaminated groundwater. Tools available to aid with screening, selecting, and implementing ISCO for remediation of contaminated groundwater, include: | ||
#ISCO e-protocol for Site-Specific Engineering & Technology Application ([https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200623/ER-200623 ESTCP Project ER-0623]<ref name="ESTCP2010"/>, based on Siegrist et al., 2011<ref name="Siegrist2011"/>) | #ISCO e-protocol for Site-Specific Engineering & Technology Application ([https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-200623/ER-200623 ESTCP Project ER-0623]<ref name="ESTCP2010"/>, based on Siegrist et al., 2011<ref name="Siegrist2011"/>) | ||
| − | #Database for ISCO ([ | + | #Database for ISCO ([[Media:DISCO.pdf|DISCO]])<ref>ESTCP, 2009. Database for ISCO (DISCO). ER-0623. [[Media:DISCO.pdf|ER-0623]]</ref> |
#ISCO Spreadsheet Design Tool ([https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626/ER-200626 CDISCO], based on Borden et al., 2010<ref>Borden, R., Cha, K.Y., Simpkin, T. and Lieberman, M.T, 2010. Development of Design Tools for Planning Aqueous Amendment Injection Systems. Permanganate Design Tool. ESTCP Project ER-200626. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626/ER-200626 ER-200626]</ref>) | #ISCO Spreadsheet Design Tool ([https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626/ER-200626 CDISCO], based on Borden et al., 2010<ref>Borden, R., Cha, K.Y., Simpkin, T. and Lieberman, M.T, 2010. Development of Design Tools for Planning Aqueous Amendment Injection Systems. Permanganate Design Tool. ESTCP Project ER-200626. [https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200626/ER-200626 ER-200626]</ref>) | ||
Revision as of 14:55, 3 May 2018
Chemical Oxidation is an in situ remediation technology that can be applied to groundwater or soils and many different contaminants. It is a chemical technology where strong oxidants are injected or mechanically mixed into the treatment zone to promote destructive abiotic degradation reactions. It is commonly used, applicable to many hydrogeologic settings, and relies on well-known technologies such as injection and mixing. Because of stoichiometry and mass balance limitations, it may be inefficient when applied to treat free-phase (i.e., free-product or non-aqueous phase liquid (NAPL)) zones.
Related Article(s):
- Chemical Oxidation Oxidant Selection (In Situ - ISCO)
- Chemical Oxidation Design Considerations(In Situ - ISCO)
- Injection Techniques for Liquid Amendments
- Monitored Natural Attenuation (MNA)
CONTRIBUTOR(S): Dr. Michelle Crimi
Key Resource(s):
Introduction
In situ chemical oxidation (ISCO) is a mature technology for remediation of contaminated groundwater, including both source zones and contaminant plumes. ISCO involves the introduction of chemical oxidants into the subsurface to react with contaminants to convert them into less harmful products. Commonly used oxidants include Fenton’s reagent, ozone, potassium permanganate, and sodium persulfate.
Treatment objectives for ISCO have ranged from reducing contaminant mass within a source zone to meeting maximum contaminant levels (MCLs) in a plume. The effectiveness of ISCO varies as it is highly dependent on proper site characterization, ISCO design considerations, and oxidant delivery system design[2][3][4][5][6][7].
One study evaluated the performance of 70 chemical oxidation projects at chlorinated solvent sites in terms of the reduction in source concentrations before and after treatment[7]. Figure 1 shows change in geometric means of parent compound (left panel) and change in geometric means of Total CVOC compound concentrations (right panel) as a result of ISCO treatment. Each symbol is an individual in situ remediation project at a specific site. The geometric mean of the before-treatment zone concentration is shown on the X-axis, and the after-treatment zone concentration is shown on the Y-axis. The different colored symbols represent different ISCO technology subtypes. The median project was able to reduce the parent compound concentrations in the treatment zone by ~84% (0.8 Orders of Magnitude or OoMs).
Contaminant Treatability
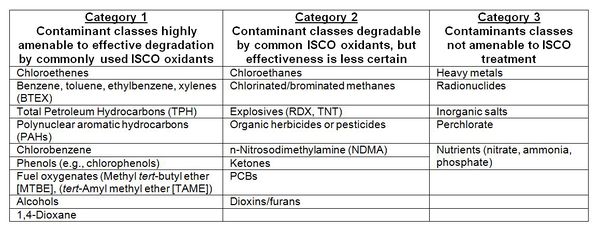
Most of the common organic contaminants can be destroyed by one or more of the oxidants. ISCO has been primarily used to treat organic chemicals, including chlorinated solvents and fuel and petroleum hydrocarbons. There are other contaminant types, though, for which ISCO is not recommended (Table 1[6]).
Applicability of ISCO to Site Conditions
Like nearly all in situ technologies, ISCO is most effective in a target treatment zone that is permeable and has a relatively low degree of heterogeneity. Prospective target zones will often be identified in existing documents and data for the site (e.g., geologic cross sections, stratigraphic representations, contaminant distribution profiles). Conditions that tend to be well-suited to ISCO generally include:
- Moderately saturated hydraulic conductivities (e.g., saturated hydraulic conductivity Ksat > 1 x 10-4 cm/s)
- Low natural organic matter content (e.g., < 0.1% dry wt.)
- Low content of reduced metals that are sensitive to changes in oxidation-reduction (redox) potential
Site conditions that tend to be challenging for effective application of ISCO include those conditions that are challenging for most in situ technologies. Key challenges are associated with:
- Strongly reducing conditions which exert high demand for some oxidants (e.g., highly reducing conditions, high organic matter, carbonates)
- Significant NAPL contaminant mass – particularly if there are extensive pools or mass trapped in zones of fractured rock
- Stringent treatment goals (i.e., goals that cannot be met by most treatment technologies) set for difficult site geology and contaminant conditions (e.g., treatment to MCLs in NAPL source zones located in a heterogeneous subsurface region)
Often these conditions that challenge ISCO as a stand-alone technology can be overcome through an ISCO treatment train approach (e.g., ISCO followed by in situ monitored natural attenuation). Also, ISCO typically requires a targeted second or third oxidant delivery event to manage contaminant rebound, which is common following single injection events.
Tools
Siegrist et al. (2011)[6] provides a comprehensive description of principles and practices of ISCO for remediation of contaminated groundwater. Tools available to aid with screening, selecting, and implementing ISCO for remediation of contaminated groundwater, include:
- ISCO e-protocol for Site-Specific Engineering & Technology Application (ESTCP Project ER-0623[1], based on Siegrist et al., 2011[6])
- Database for ISCO (DISCO)[8]
- ISCO Spreadsheet Design Tool (CDISCO, based on Borden et al., 2010[9])
Advantages and Disadvantages
All remediation technologies have potential advantages and disadvantages depending on site conditions, contaminant conditions, and clean-up goals. ISCO has the same potential advantages and disadvantages that are inherent to in situ remediation technologies, as well as some that are unique to chemical oxidation. Advantages include:
- Robust treatment method
- Can be implemented quickly
- Variety of oxidants and activation approaches
- Variety of delivery approaches
- Applicable to a range of subsurface conditions
- Relatively low mobilization costs
- Ability to couple with pre- and post-treatment methods
- Generally well-accepted by the regulatory community
Potential disadvantages include:
- Potential need for large amounts of chemical
- Resistance of some contaminants to oxidation
- Limited ability to penetrate low permeability soil and groundwater zones
- Potential for ISCO-induced effects (e.g., gas evolution, permeability reduction, secondary water quality effects)
- Potential for rebound of target contaminants
- Inability to treat contaminant source zones to the most stringent goal levels (e.g., MCLs)
Summary
Successful ISCO treatment requires matching an oxidant to the contaminant of concern and site conditions, using an effective delivery approach, and sustaining an adequate concentration of oxidant for a sufficient period of time so contaminant oxidation can occur. It is also very important to consider health and safety while planning and implementing ISCO. ISCO oxidants are strong oxidizing agents. Workers must be properly trained and equipped with proper protective equipment, ISCO operations must be carefully monitored, and oxidants must be properly stored.
References
- ^ 1.0 1.1 Siegrist, R.L., 2010. In situ chemical oxidation for groundwater remediation - technology practices manual. ESTCP Project ER-0623. ER-200623
- ^ Siegrist, R. L. Urynowicz, M.A., West, O.R., Crimi, M.L. and Lowe, K.S., 2001. Principles and practices of in situ chemical oxidation using permanganate. Columbus, OH: Battelle Press. ISBN-10: 1574771027. doi: 10.1016/S0304-3894(01)00355-7
- ^ ITRC, 2005. Technical and Regulatory Guidance for In Situ Chemical Oxidation of Contaminated Soil and Groundwater. Council TITaR, editor. Report pdf
- ^ Huling, S. G., and Pivetz, B. E., 2006. In-situ chemical oxidation (No. EPA/600/R-06/072). Environmental Protection Agency, Washington, DC. Office of Water. Report pdf
- ^ Krembs, F.J., Siegrist, R.L., Crimi, M.L., Furrer, R.F. and Petri, B.G., 2010. ISCO for groundwater remediation: analysis of field applications and performance. Groundwater Monitoring & Remediation, 30(4), 42-53. doi: 10.1111/j.1745-6592.2010.01312.x
- ^ 6.0 6.1 6.2 6.3 6.4 Siegrist, R.L., Crimi, M. and Simpkin, T.J. eds., 2011. In situ chemical oxidation for groundwater remediation (Vol. 3). Springer Science & Business Media. 678 pgs. ISBN: 978-1-4419-7825-7. doi: 10.1007/978-1-4419-7826-4
- ^ 7.0 7.1 McGuire, T., 2016. Development of an Expanded, High-Reliability Cost and Performance Database for In-Situ Remediation Technologies. ESTCP Project ER-201120. ER-201120
- ^ ESTCP, 2009. Database for ISCO (DISCO). ER-0623. ER-0623
- ^ Borden, R., Cha, K.Y., Simpkin, T. and Lieberman, M.T, 2010. Development of Design Tools for Planning Aqueous Amendment Injection Systems. Permanganate Design Tool. ESTCP Project ER-200626. ER-200626
See Also
- Improved Understanding of Fenton-Like Reactions for In Situ Remediation of Contaminated Groundwater Including Treatment of Sorbed Contaminants and Destruction of DNAPLs
- Improved Understanding of In Situ Chemical Oxidation (ISCO)
- Reaction and Transport Processes Controlling In Situ Chemical Oxidation of DNAPLs
- Control of Manganese Dioxide Particles Resulting from In Situ Chemical Oxidation Using Permanganate
- Enhanced Reactant-Contaminant Contact Through the Use of Persulfate In Situ Chemical Oxidation (ISCO)
- Semi-Passive Oxidation-Based Approaches for Control of Large, Dilute Groundwater Plumes of Chlorinated Ethylenes
- Impacts on Groundwater Quality Following the Application of ISCO: Understanding the Cause of and Designing Mitigation for Metals Mobilization
- CleanOX® In Situ Chemical Oxidation of Groundwater
- Remediation of DNAPL through Sequential In Situ Chemical Oxidation and Bioaugmentation
- In Situ Chemical Oxidation for Groundwater Remediation - Technology Practices Manual
- Biological Oxidation of Dichloroethene through Manganese Addition
- Field Demonstration, Optimization, and Rigorous Validation of Peroxygen-Based ISCO for the Remediation of Contaminated Groundwater
- Cooperative Technology Demonstration: Polymer-Enhanced Subsurface Delivery and Distribution of Permanganate