Chemical Oxidation Oxidant Selection (In Situ - ISCO)
In situ chemical oxidation (ISCO) is a soil and/or groundwater remediation technology that introduces chemical oxidants into contaminated subsurfaces in order to react with contaminants, resulting in the conversion of the contaminants into less harmful products. Various oxidants are available for ISCO projects that have different chemical properties, oxidation potential, and delivery systems that can be applied to particular site-specific conditions. A variety of factors should be considered when selecting an oxidant, including geochemistry, contaminant type, and injection options.
Related Article(s):
CONTRIBUTOR(S): Dr. Michelle Crimi
Key Resource(s):
- In Situ Chemical Oxidation[1]
- In Situ Chemical Oxidation for Groundwater Remediation Technology Practices Manual[2]
Introduction
Chemical oxidants are introduced into contaminated subsurfaces in order to initiate chemical reactions, causing oxidative breakdowns through electron transfer processes (gain or loss of electrons resulting from transformation) or through the generation (via use of activating chemicals or materials) of free radical species. Permanganate, catalyzed hydrogen peroxide, persulfate, and ozone (discussed below) are the most common oxidants used in ISCO (Table 1[1]). However, peroxone, percarbonate, and calcium peroxide are also viable.
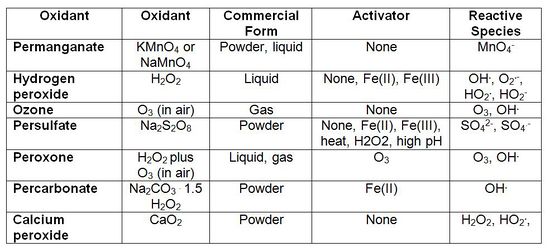
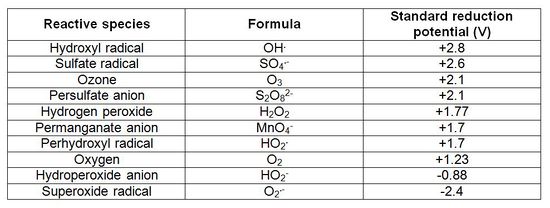
Oxidants behave in different ways. Permanganate and unactivated persulfate oxidants breakdown through electron transfer while catalyzed hydrogen peroxide and activated persulfate oxidants breakdown through the generation of free radicals. Ozone reactions can occur through both electron transfer and free radical formation. The need to activate oxidants to generate free radicals and the sensitivity of treatment to matrix conditions, such as temperature and pH, vary with the different oxidants and specific contaminants (Table 2[1]).
Common Oxidants
Here, we summarize the basic processes associated with the more common oxidants:
Permanganate
Permanganate (either sodium or potassium permanganate; NaMnO4 or KMnO4) reactions occur by transfer of electrons between the oxidant and contaminants. Reactions are only applicable to certain types of organic compounds. Permanganate is more reactive with compounds containing alkene carbon-carbon double bonds than with saturated hydrocarbons (compounds with carbon-carbon single bonds). Permanganate reactions are less dependent on pH then catalyzed hydrogen peroxide systems. Manganese dioxide solids are a byproduct of permanganate reactions that have the potential to reduce formation permeability, particularly if the oxidant is used at high concentration for treating contaminants present at high mass density. Permanganate reacts with naturally occurring reduced materials in the subsurface – this reaction is termed natural oxidant demand (NOD). The rate of NOD must be considered in system design as it can interfere with target contaminant destruction.
Catalyzed Hydrogen Peroxide
Catalyzed hydrogen peroxide, also sometimes referred to as Fenton’s reagent, is a strong oxidant with a history of application in industry and water treatment. A wide variety of reactive free radicals, including hydroxyl radical (OH.), superoxide radical (O2.), and perhydroxyl radical (HO2.), result when hydrogen peroxide is catalyzed. The specific radicals that result depend on solution pH, catalysts used, and the concentration of oxidant used. For groundwater remediation, the most common approaches for catalyzing hydrogen peroxide include ferrous and ferric iron, iron and manganese soil minerals, and other metals in solution. These catalysts may be naturally present at a site or added during ISCO. The lifetime of hydrogen peroxide in the subsurface is short, generally hours to days. Its reactive transport in the subsurface is limited because of this fast rate of reaction.
Ozone
Ozone is a gaseous oxidant that is very reactive and short-lived. Ozone is generated onsite, then immediately injected into the subsurface, typically by sparging. Reactions are through free radical or electron transfer processes and many contaminant types are amenable to reaction with ozone. Because ozone is a gas, it has an advantage over liquid amendments for treating contaminants in the vadose zone (above the groundwater table). Ozone is typically generated and injected over longer timeframes than other oxidants, depending on other site-specific conditions.
Persulfate
Persulfate (typically sodium persulfate; Na2S2O8) reaction chemistry is complex – it can react through electron transfer or free radical processes. When activated, free radicals are formed, which are reactive with a wide array of contaminants. Sulfate radical and hydroxyl radical are primarily credited with having the major role in reactions with contaminants; however, evidence continues to emerge that other free radicals, such as superoxide and perhydroxyl radical, may also be important. Activation by natural media constituents is site specific and our understanding of the influence of natural media mineralogy and organic carbon on persulfate activation continues to advance. Activation can also be achieved during persulfate ISCO by elevating pH to >11, heat-activating the persulfate, adding ferrous or ferric iron, or by combining persulfate and hydrogen peroxide (both are activated/catalyzed).
Contaminant Treatability
Oxidant reactivity and contaminant treatability by different oxidants varies by contaminant properties, the presence of mixed contaminants, and the specific subsurface characteristics. In general, all petroleum hydrocarbons and most chlorinated solvents can be treated by ISCO although the effectiveness differs by compound. Ethenes are amenable to treatment by all oxidants, depending on site-specific conditions. More recalcitrant contaminants, such as chlorinated ethanes, require more aggressive, free-radical based oxidants such as catalyzed hydrogen peroxide, activated persulfate, or ozone. Other factors that affect contaminant treatability are site-specific, and include pH, alkalinity, site soil organic carbon content, and contaminant concentration. In general, sites with high contaminant concentrations and high soil organic carbon content are challenging to treat with any oxidant, while pH and alkalinity affect oxidants differently. Detailed guidance and decision matrices for selecting an appropriate oxidant for contaminant and site-specific properties exist[3]. An electronic tool for oxidant selection for site-specific conditions is available[2].
Treatability Testing
Laboratory testing can be conducted to help choose the most effective oxidant for each site. Testing can determine:
- Oxidant demand and oxidant persistence (rate and extent of depletion)
- Permanganate NOD - 48-hr test in American Society for Testing and Materials (ASTM) method D7262-07 for sites where permanganate is a likely candidate
- Permanganate rate of depletion test protocol: Attachment 16 of the ESTCP Project ER-0623[2]
- Contaminant or co-contaminant degradability and potential for intermediates and/or byproducts, or for metals solubilization (resulting from changes in pH and oxidation-reduction potential (ORP))
- Bench testing protocol for contaminant treatability and byproducts: Attachment 17 of the ESTCP Project ER-0623[2]
- Contaminant desorption or dissolution
- Optimized oxidant dose or activation approach
Laboratories that conduct such testing typically (a) evaluate rates and/or extents of contaminant destruction and oxidant depletion with ranges of oxidants, oxidant activation/catalysis, and oxidant dose, and then (b) measure contaminant, oxidant, anticipated byproducts, and other factors such as pH and temperature, as appropriate, over time. Laboratory systems typically have turbulent mixing and achieve 100% contact between soil, water, contaminant, and oxidant with no dilution or dispersion. Therefore, contaminant destruction results present a best case scenario, whereas oxidant depletion results offer a worst case scenario.
Field pilot testing is typically conducted to evaluate scale-dependent processes that impact ISCO such as the ability of the formation to accept a volume of oxidant or rate of delivery, the radius of influence of oxidant delivered, and the impact of heterogeneities on oxidant distribution. It may be possible to get a sense for overall treatment effectiveness, but site heterogeneities and uncertainties associated with upscaling system design should be considered. Guidance for field pilot-scale testing is available as Attachment 18 of the ESTCP Project ER-0623[2].
Summary
There are a number of variants for implementing chemical oxidation starting with the selection of an oxidant. Different oxidants have different chemical properties, oxidation potential, and delivery systems that can be applied to particular site specific conditions.
References
- ^ 1.0 1.1 1.2 1.3 1.4 Huling, S. G., and Pivetz, B. E., 2006. In-situ chemical oxidation (No. EPA/600/R-06/072). Environmental Protection Agency, Washington, DC. Office of Water. Report pdf
- ^ 2.0 2.1 2.2 2.3 2.4 Siegrist, R.L., 2010. In situ chemical oxidation for groundwater remediation - technology practices manual. ESTCP Project ER-0623. ER-200623
- ^ Siegrist, R.L., Crimi, M. and Simpkin, T.J. eds., 2011. In situ chemical oxidation for groundwater remediation (Vol. 3). Springer Science & Business Media. 678 pgs. ISBN: 978-1-4419-7825-7. doi: 10.1007/978-1-4419-7826-4